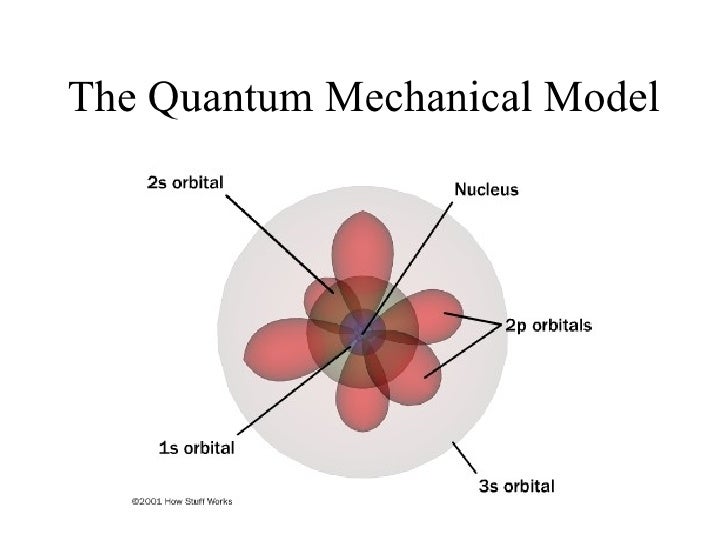
The Quantum Mechanical Model was created by the Austrian Physicist, Erwin Schrödinger in 1926. Erwin Schrödinger is well know for is theory of observation, based of his Schrödinger's cat experiment. The Quantum Mechanical Model uses a mathematical equation to predict the likelihood of an electron being in a certain position. This model can be portrayed as a grouping of electron clouds around a nucleus, with different density levels corresponding to the probability of an electron being there. Schrödinger's Model is similar to the Bohr's model, except it doesn't define the exact path of an electron, but rather predict the odds of the location of an electron. This model also introduces the concept of sub-energy levels.
"Development of the Atomic Theory." Development of the Atomic Theory. ABCTE, 2007. Web. 04 Oct. 2015. <http://www.abcte.org/files/previews/chemistry/s1_p6.html>.
No comments:
Post a Comment