In 1913 Niels Bohr came to work in the laboratory of Ernest Rutherford. Rutherford, who had a few years earlier, discovered the planetary model of the atom asked Bohr to work on it. Soon after, he came up with the Bohr model of the atom. The Bohr model was based off Rutherford's model, but with he suggested that electrons assume only certain orbits around the nucleus. Each orbit was then stated to have an energy level associated with it. For example the orbit closest to the nucleus has an energy E1, the next closest E2 and so on. These energy levels are called excited states of the electrons, with E1 being the ground state.
Leon, N. De, Prof. "Bohr Model of the Atom." Bohr Model of the Atom. Indiana University, n.d. Web. 04 Oct. 2015. <http://www.iun.edu/~cpanhd/C101webnotes/modern-atomic-theory/Bohr-model.html>.
Search This Blog
Sunday, October 4, 2015
Ernest Rutherford
In 1911 Ernest Rutherford performed an experiment to test the plum pudding model. He fired energetic a [He2+] particles at a foil, and measured the deflection of the particles as they came out the other side. Rutherford expected all of the particles to be deflected just a bit as they passed through the "plum pudding". He found that most of the particles he shot at the foil were not deflected at all.Rutherford's result lead him to believe that most of the foil was made of empty space, but had extremely small, dense lumps of matter inside. His experiment led to the discovery of the nucleus. The problems was according to the physics of the time Rutherford's planetary atom should have an extremely short lifetime.
"The Rutherford Experiment." The Rutherford Experiment. The State University of New Jersey Department of Physics & Astronomy, n.d. Web. 04 Oct. 2015. <http://www.physics.rutgers.edu/~tianfeng/meis_1205/Rutherford.htm>.
"The Rutherford Experiment." The Rutherford Experiment. The State University of New Jersey Department of Physics & Astronomy, n.d. Web. 04 Oct. 2015. <http://www.physics.rutgers.edu/~tianfeng/meis_1205/Rutherford.htm>.
J.J. Thompson
In 1897 the British physicist Joseph John Thomson discovered the electron through a series of experiments using a cathode-ray tube. He studied the behavior of electric discharge in a high-vacuum cathode-ray tube, and he interpreted the deflection of the rays by electrically charged plates and magnets as evidence of electrons. In 1904 Thomson contributed his version of the atomic model as a sphere of positive matter in which electrons are positioned by electrostatic forces. His atomic model is called the plum pudding model because he predicted the positive and negative particles balanced each other out without an pattern or structure, because the nucleus had not been discovered.
"Joseph John Thomson | Chemical Heritage Foundation." Joseph John Thomson | Chemical Heritage Foundation. Chemical Heritage Foundation, n.d. Web. 04 Oct. 2015. <http://www.chemheritage.org/discover/online-resources/chemistry-in-history/themes/atomic-and-nuclear-structure/thomson.aspx>.
"Joseph John Thomson | Chemical Heritage Foundation." Joseph John Thomson | Chemical Heritage Foundation. Chemical Heritage Foundation, n.d. Web. 04 Oct. 2015. <http://www.chemheritage.org/discover/online-resources/chemistry-in-history/themes/atomic-and-nuclear-structure/thomson.aspx>.
John Dalton
John Dalton was an english chemist known best for his Atomic Theory. Dalton's Theory consisted of three main points. First off, Dalton stated that each element is composed of extremely small particles, too small to be seen by the naked eye. These particles are called atoms, which Dalton theorized could never be created or destroyed. His second point was that all atoms of the same element are alike in mass and other properties, but the atoms of other elements differ from all other elements. Finally, his third point was that for each compound, different elements combine in a simple numerical ratio. Dalton based his points off the Law of Conservation of Mass and the Law of Constant Composition.
Dalton's second and third points have since been proven incorrect. His first point proven incorrect when scientist split an atom through nuclear fission, and his second was proven incorrect when isotopes were discovered.
Larsen, Delmar. "Atomic Theory." - Chemwiki. N.p., 02 Oct. 2013. Web. 04 Oct. 2015. <http://chemwiki.ucdavis.edu/Physical_Chemistry/Atomic_Theory/Atomic_Theory>.
Dalton's second and third points have since been proven incorrect. His first point proven incorrect when scientist split an atom through nuclear fission, and his second was proven incorrect when isotopes were discovered.
Larsen, Delmar. "Atomic Theory." - Chemwiki. N.p., 02 Oct. 2013. Web. 04 Oct. 2015. <http://chemwiki.ucdavis.edu/Physical_Chemistry/Atomic_Theory/Atomic_Theory>.
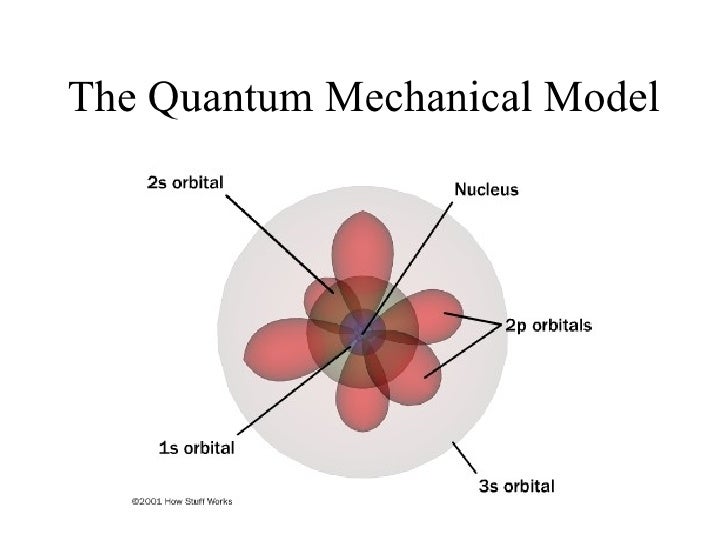
The Quantom Mechanical Model
The Quantum Mechanical Model was created by the Austrian Physicist, Erwin Schrödinger in 1926. Erwin Schrödinger is well know for is theory of observation, based of his Schrödinger's cat experiment. The Quantum Mechanical Model uses a mathematical equation to predict the likelihood of an electron being in a certain position. This model can be portrayed as a grouping of electron clouds around a nucleus, with different density levels corresponding to the probability of an electron being there. Schrödinger's Model is similar to the Bohr's model, except it doesn't define the exact path of an electron, but rather predict the odds of the location of an electron. This model also introduces the concept of sub-energy levels.
"Development of the Atomic Theory." Development of the Atomic Theory. ABCTE, 2007. Web. 04 Oct. 2015. <http://www.abcte.org/files/previews/chemistry/s1_p6.html>.
"Development of the Atomic Theory." Development of the Atomic Theory. ABCTE, 2007. Web. 04 Oct. 2015. <http://www.abcte.org/files/previews/chemistry/s1_p6.html>.
Subscribe to:
Posts (Atom)